TFMLA
New Compound for Fundamental Studies in Chirality
(S)-3,3,3-Trifluoro-2-Hydroxy-2-Methylpropanoic Acid
(a-Trifluoromethyl Lactic Acid, “Soloshonok Acid”)
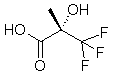
>99.9% ee
(S)-TFMLA
009358
It is commonly known, that enantiomerically pure compounds and their corresponding racemic counterparts, have significantly different physical properties, such as melting point and solubility [1]. On the other hand, differences between enantiopure and racemic compounds in the rates of their sublimation have been overlooked and remain virtually unstudied. Quite recently however, this area of research has received substantial practical and theoretical attention [2]. Major points of interest in studying the physicochemical aspects of sublimation of enantiopure and racemic compounds include: a) an assumption that sublimation should be reasonably regarded as a plausible mechanism for the formation of optically active crystals [3]; b) rational application of simple sublimation for enantiomeric purifications can be the next new, non-conventional and ultimately economical practical method for preparation of chiral compounds in enantiomerically pure form [4].
One of the most desirable model compounds to study the phenomenon of different sublimation rates between racemic and enantiomerically pure compounds is (S)-TFMLA [5] and its various derivatives [6]. While some physicochemical [7] and theoretical [8] investigations using (S)-TFMLA has been already conducted, many intellectually intriguing questions about this compound and its derivatives still await comprehensive study.
While the racemic form of TFMLA is commercially available, Oakwood offers enantiomerically pure (>99.9% ee) S)-TFMLA (#009358) for further studies of unique properties of this compound.
References:
[1] J. Jacques, A. Collet and S. H. Wilen, Enantiomers, Racemates, and Resolutions, Wiley, New York, 1981.
[2] (a) S. P. Fletcher, R. B. C. Jagt and B. L. Feringa, Chem. Commun., 2007, 2578; (b) R. H. Perry, C. Wu, M. Nefliu and R. G. Cooks, Chem. Commun., 2007, 1071; (c) A. Bellec and J.-C. Guillemin, Chem. Commun., 2010, 46, 1482.
[3] Cintas, P. Angew. Chem. Int. Ed. 2008, 47, 2918-2920.
[4] Ueki, H.; Yasumoto, M.; Soloshonok V. A. Tetrahedron: Asymmetry, 2010, 21, 1396.
[5] Soloshonok, V. A; Ueki, H.; Yasumoto, M.; Mekala, S.; Hirschi, J. S.; Singleton, D. A. J. Am. Chem. Soc. 2007, 129, 12112-12113.
[6] Yasumoto, M.; Ueki, H. Soloshonok V. A. J. Fluor. Chem. 2010, 131, 540-544.
[7] Albrecht, M.; Soloshonok, V. A.; Schrader, L.; Yasumoto, M.; Suhm, M. A. J. Fluor. Chem. 2010, 131, 495-504.
[8] (a) Tsuzuki, S.; Orita, H.; Ueki, H.; Soloshonok, V. A. J. Fluor. Chem. 2010 131, 461-466; (b) Tonner, R.; Soloshonok, V. A.; Schwerdtfeger, P. Phys. Chem. Chem. Phys., 2010, DOI: 10.1039/C0CP01155J.